Complete Guide to Patient Monitoring for Dental Implant Surgery: CO₂ Monitoring, Safety Protocols & Equipment Selection
Patient monitoring during dental implant surgery and sedation procedures is not just a regulatory requirement—it's a critical component of patient safety that can mean the difference between a successful procedure and a medical emergency.
As dental practices increasingly offer IV sedation and complex surgical procedures like full-arch implant placement, understanding the proper patient monitoring equipment and protocols has become essential. This comprehensive guide will walk you through everything you need to know about selecting and implementing patient monitoring systems in your dental practice.
Why CO₂ Monitoring Is Non-Negotiable for Dental Sedation
Carbon dioxide (CO₂) monitoring, also known as capnography, has become the gold standard for patient monitoring during dental sedation procedures. Unlike pulse oximetry, which provides a delayed indication of respiratory problems, CO₂ monitoring offers real-time detection of airway obstruction and respiratory depression—the two most common complications during sedation.
The American Dental Association (ADA) and many state dental boards now recommend or require continuous CO₂ monitoring for moderate and deep sedation procedures. This requirement has driven many practices to upgrade from basic vital signs monitors to comprehensive patient monitoring systems with integrated capnography capabilities.
For high-volume implant practices performing procedures under IV sedation, a Mindray ePM 12MA patient monitor with CO₂ monitoring provides the comprehensive monitoring capabilities needed to detect respiratory issues before they become critical emergencies.
Essential Monitoring Parameters for Dental Implant Surgery
When performing dental implant surgery with sedation, your patient monitoring system should track these critical parameters:
- End-Tidal CO₂ (EtCO₂): Measures the concentration of carbon dioxide at the end of each breath, providing immediate feedback on ventilation adequacy
- Oxygen Saturation (SpO₂): Monitors blood oxygen levels to detect hypoxemia
- Heart Rate & ECG: Tracks cardiac function and rhythm abnormalities
- Blood Pressure (NIBP): Non-invasive blood pressure monitoring to detect cardiovascular changes
- Respiratory Rate: Monitors breathing frequency to identify respiratory depression
Different procedures require different levels of monitoring sophistication. A simple extraction with minimal sedation might only need basic vital signs tracking with a Mindray VS8 vital signs monitor, while complex full-arch implant cases under deep sedation demand comprehensive multi-parameter monitoring with CO₂ capabilities.
Choosing the Right Patient Monitor for Your Practice Type
The patient monitoring equipment your practice needs depends on several factors: the types of procedures you perform, your sedation protocols, state regulations, and your practice's volume and growth plans.
For High-Volume Implant Practices: If your practice performs multiple implant cases weekly with IV sedation, you need a robust monitor that can handle continuous use. The Mindray ePM 12MA with its 12-inch display and CO₂ monitoring provides the visibility and functionality needed in busy surgical environments. Its large screen allows the entire surgical team to monitor patient status at a glance, while the integrated CO₂ module meets regulatory requirements without requiring additional equipment.
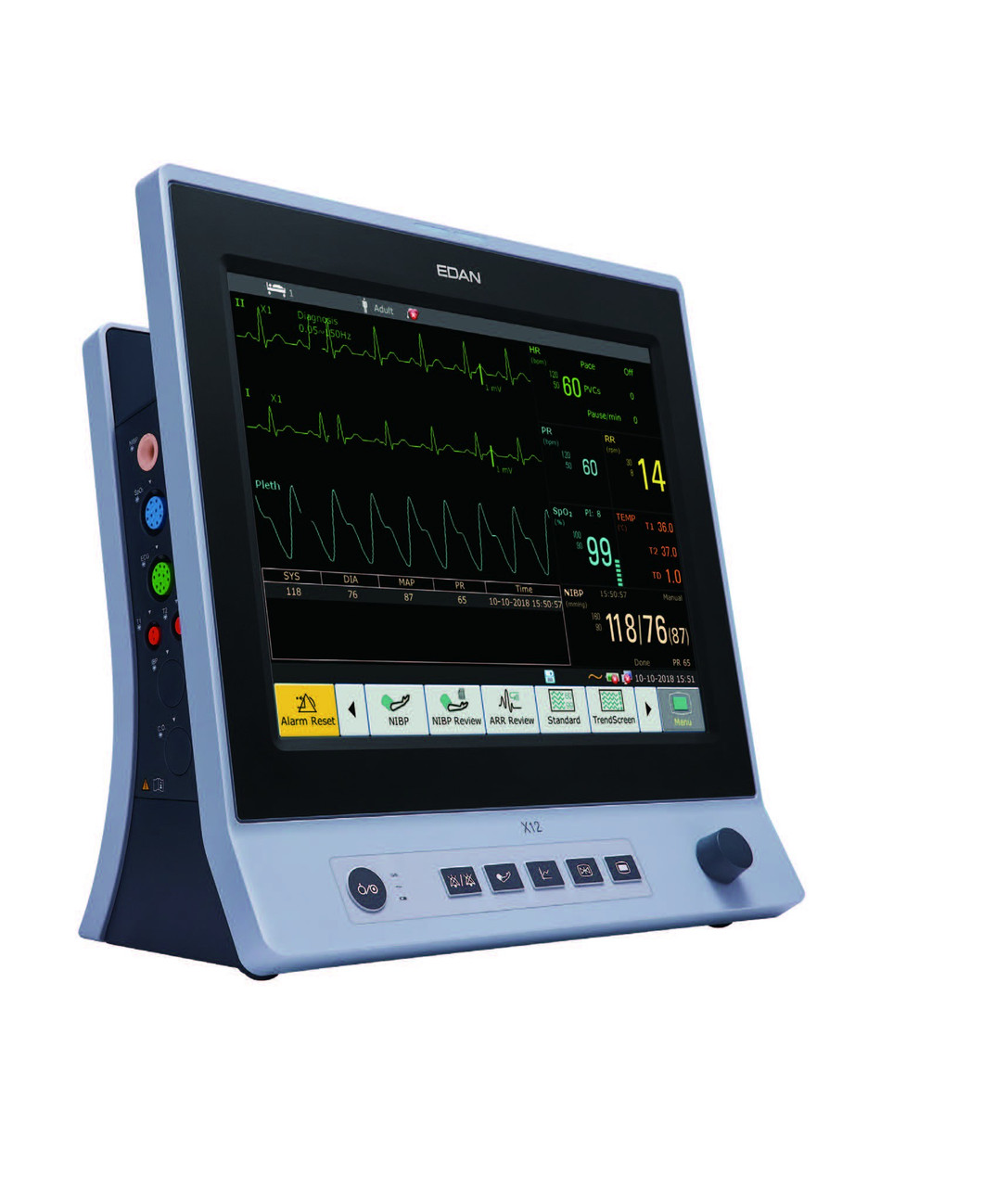
For Oral Surgery Practices: Oral surgeons performing extractions, bone grafts, and moderate sedation procedures benefit from monitors with touchscreen efficiency and portability. The Edan iM Series patient monitors offer an excellent balance of functionality and ease of use, with intuitive touchscreen interfaces that make patient data recording and review quick and efficient during busy surgical days.
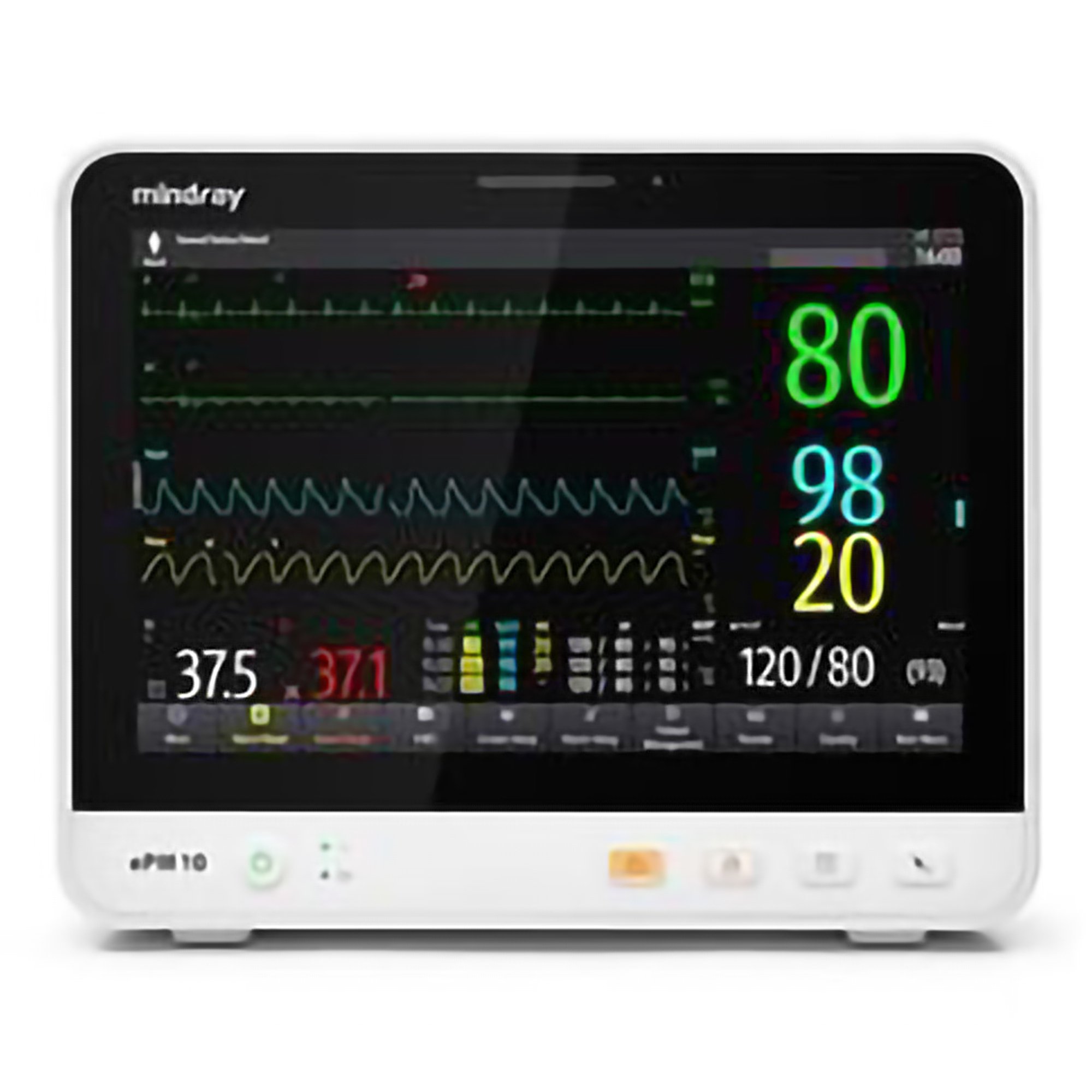
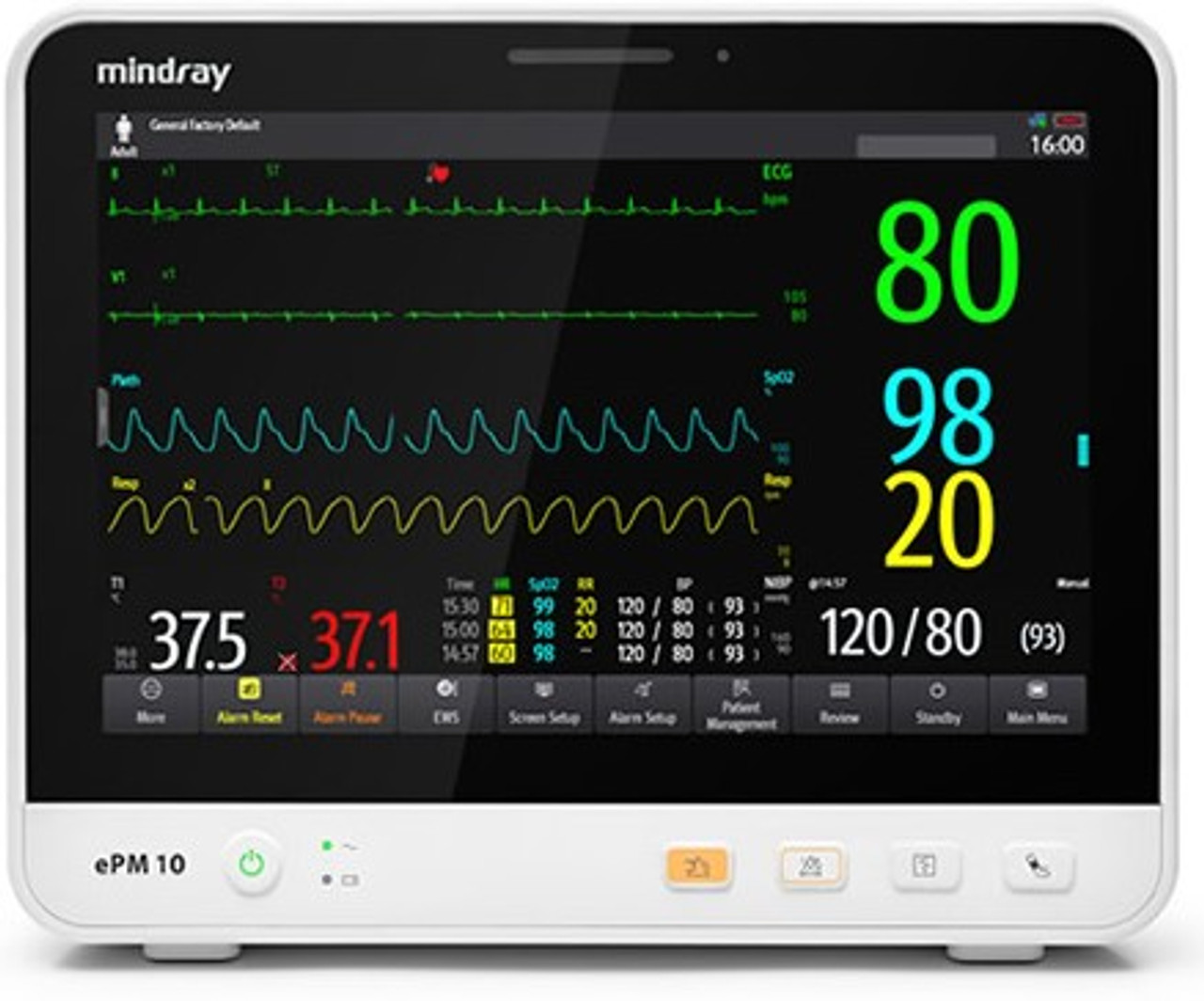
For General Dentists Expanding Services: If you're a general dentist adding implant placement or sedation services to your practice, you need equipment that meets credentialing requirements while remaining budget-conscious. The Mindray ePM 10A patient monitor provides essential CO₂ monitoring and multi-parameter tracking at a more accessible price point, making it ideal for practices transitioning into sedation dentistry.
Understanding CO₂ Waveform Capnography vs. Basic Monitoring
Not all CO₂ monitoring is created equal. There's a critical difference between colorimetric CO₂ detection (which simply indicates the presence of CO₂) and continuous waveform capnography (which provides detailed, real-time breath-by-breath analysis).
Waveform capnography displays the complete respiratory cycle as a continuous waveform, allowing you to identify subtle changes in ventilation patterns that might indicate developing airway obstruction, inadequate ventilation, or equipment malfunction. This level of detail is increasingly required by state dental boards for moderate and deep sedation permits.
When evaluating patient monitors, ensure they provide true waveform capnography rather than just numeric EtCO₂ values. Both the Mindray ePM series and Edan iM Series offer comprehensive waveform capnography that meets regulatory requirements for dental sedation.
Patient Monitoring Protocols for Different Sedation Levels
The ADA guidelines specify different monitoring requirements based on sedation depth:
Minimal Sedation (Anxiolysis): At this level, patients remain responsive and maintain protective reflexes. Basic monitoring of vital signs including pulse oximetry and heart rate is typically sufficient. A basic vital signs monitor meets requirements for most minimal sedation procedures.
Moderate Sedation (Conscious Sedation): This is where CO₂ monitoring becomes critical. Patients are less responsive, and the risk of respiratory depression increases significantly. Continuous monitoring of oxygenation, ventilation (via CO₂), circulation, and blood pressure is required. Most state boards require CO₂ monitoring at this level.
Deep Sedation: At deep sedation levels, patients may not respond purposefully and cannot maintain airway patency independently. Comprehensive multi-parameter monitoring with continuous CO₂ waveform capnography is mandatory. This level of sedation requires the most sophisticated monitoring equipment and trained personnel.
Common Patient Monitoring Mistakes to Avoid
Even with proper equipment, certain monitoring errors can compromise patient safety:
- Relying solely on pulse oximetry: SpO₂ is a lagging indicator of respiratory problems. By the time oxygen saturation drops, significant respiratory depression may have already occurred. CO₂ monitoring provides much earlier warning signs.
- Inadequate staff training: Having sophisticated monitoring equipment is useless if your team doesn't understand how to interpret the data. Regular training on recognizing abnormal waveforms and vital sign patterns is essential.
- Poor alarm management: Setting alarm limits too wide (to avoid nuisance alarms) can delay recognition of actual problems. Conversely, overly sensitive alarms that constantly trigger cause alarm fatigue and may be ignored when a real emergency occurs.
- Incomplete documentation: Many state boards require periodic documentation of vital signs during sedation procedures. Ensure your monitoring equipment and protocols include proper documentation practices.
FDA Approval and Regulatory Compliance
All patient monitoring equipment used in dental practices must have FDA 510(k) clearance for medical use. This certification ensures the device meets safety and effectiveness standards for healthcare applications. When purchasing monitoring equipment, verify that it carries proper FDA clearance and is specifically approved for the type of monitoring you'll be performing.
Both Mindray and Edan, the manufacturers of the patient monitors featured on this site, maintain FDA 510(k) clearance for their medical devices. This regulatory approval is essential not only for legal compliance but also for liability protection and insurance credentialing.
Integration with Practice Management and Documentation
Modern patient monitors can integrate with practice management systems and electronic health records, streamlining documentation and reducing staff workload. When evaluating monitoring equipment, consider whether it offers data export capabilities, printable reports, or direct EMR integration.
The ability to automatically record and store vital signs data throughout a procedure not only ensures regulatory compliance but also provides valuable documentation in the rare event of a patient complication or legal inquiry. This documentation capability should be a key consideration in your equipment selection process.
Cost Considerations and Return on Investment
Patient monitoring equipment represents a significant investment, with costs ranging from $2,000 for basic vital signs monitors to $8,000+ for comprehensive multi-parameter systems with CO₂ monitoring. However, this investment pays dividends in multiple ways:
- Enables you to perform more profitable procedures requiring sedation
- Meets credentialing requirements for sedation permits and hospital affiliations
- Reduces liability exposure through proper patient monitoring
- Enhances patient safety and practice reputation
- Provides competitive advantage in your market
Many practices find that the ability to offer in-house IV sedation for implant cases—enabled by proper monitoring equipment—quickly justifies the equipment investment through increased case acceptance and revenue.
Making Your Final Equipment Decision
Selecting the right patient monitoring equipment for your practice comes down to matching your specific needs with the appropriate technology level. Consider these factors:
- Current procedure mix and sedation levels used
- Planned practice growth and service expansion
- State regulatory requirements for your sedation permit
- Number of operatories requiring monitoring equipment
- Budget constraints and financing options
- Staff technical comfort level and training needs
For most dental practices performing implant surgery with sedation, investing in a comprehensive patient monitor with CO₂ monitoring capabilities—such as the Mindray ePM 12MA or Edan iM Series—provides the safety, regulatory compliance, and professional capabilities needed for long-term success in modern implant dentistry.
Ready to Upgrade Your Patient Monitoring?
Our team can help you select the right patient monitoring equipment for your practice's specific needs, ensuring you meet all regulatory requirements while staying within budget.
Request a Custom Quote →